Biomimetic engineering and imaging
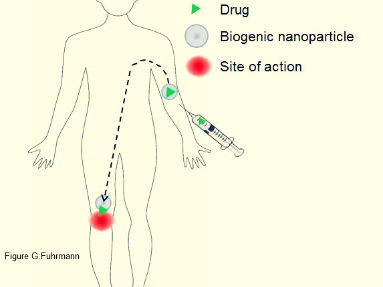
Our past and future research is chiefly focused on different ways and means on how drugs can be protected and delivered in different environments of the human body.
In particular, we are interested in the design of non-invasive, real-time detection methods and the development of innovative biomimetic drug carriers, which harness mechanisms present in nature to enable selective delivery of therapeutics.
Read more on our work here and below
Extracellular vesicles
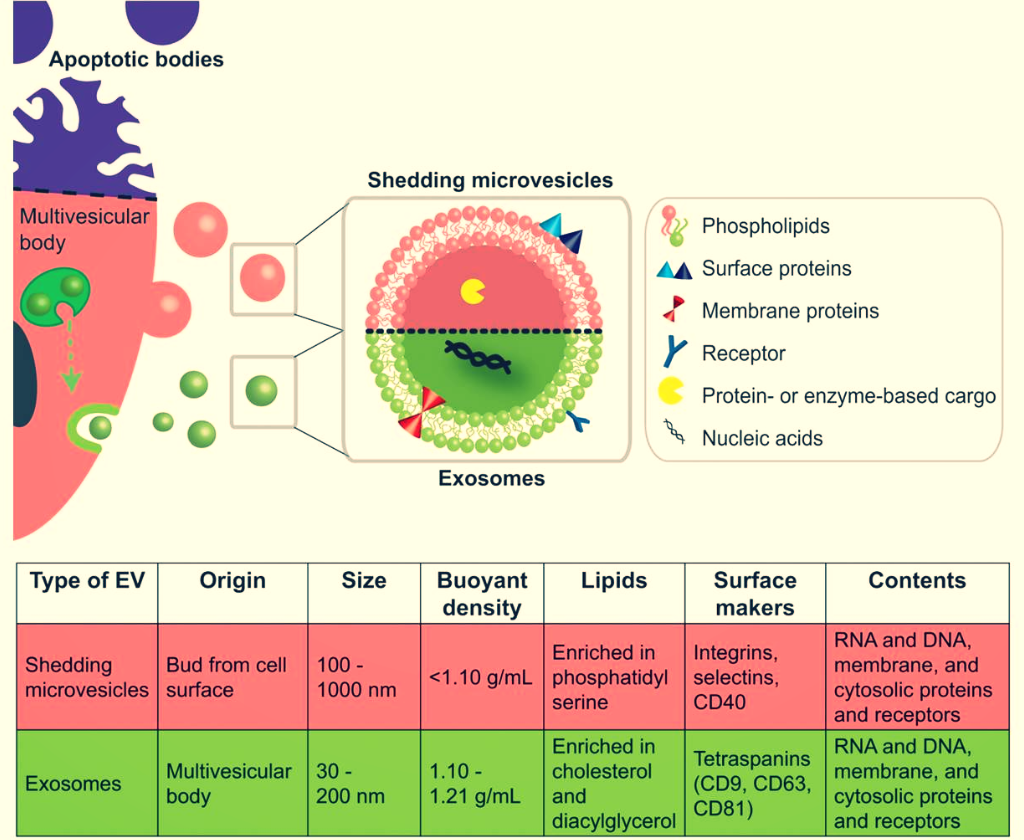
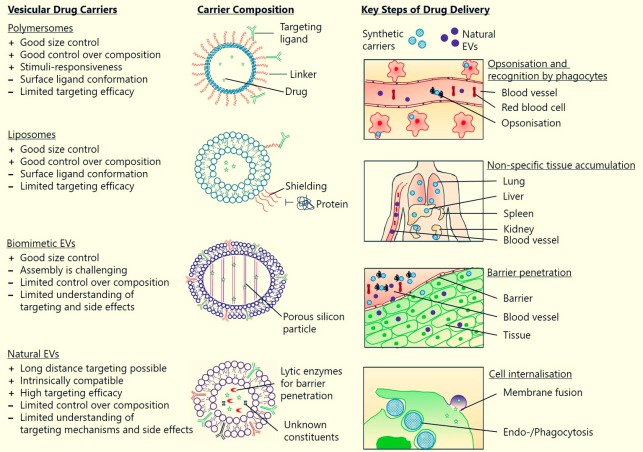
We are currently extending the idea of protecting and delivering therapeutic agents by exploring the potential of extracellular vesicles (EVs) as smart drug carrier systems. These cell-derived particles are produced by almost all cells in vitro and in vivo and they are known to be conveyors of cell-to-cell communication (Herrmann [...] Fuhrmann, Nature Nanotechnology (2021)).
In addition, EVs feature a natural composition, stability in physiological fluids and can potentially bypass immune activation rendering them promising drug transporters. Nevertheless, efficient methods to load small molecule drugs into EVs need to be explored in order to harness their exceptional properties (Fuhrmann* et al., J Control Release (2016)).
We have shown that EVs could successfully be loaded with drugs of different hydrophobicity, which represents an essential step to develop them in a therapeutic setting. Moreover, EVs are able to deliver their cargo efficient and at the cellular level indicating their significance as smart drug carriers.
We published a video manuscript in the Journal of Visualized Experiments on EV lyophilisation:
Outer membrane vesicles
Most recently, we are focussing on characterising and understanding bacterial outer membrane vesicles (OMVs). These bacterial EVs are also important key mediators of interaction between bacteria and host cells. We have discussed they diagnostic and therapeutic potential (Fuhrmann* et al., EJPB (2017)).
We aim at understanding OMVs' role during infection and how they could potentially applied for novel treatment avenues We have discovered that bacterial vesicles from soil-living myxobacteria are inherently antibiotic and they can interfere with bacterial biofilms.
Polymer-enzyme conjugates
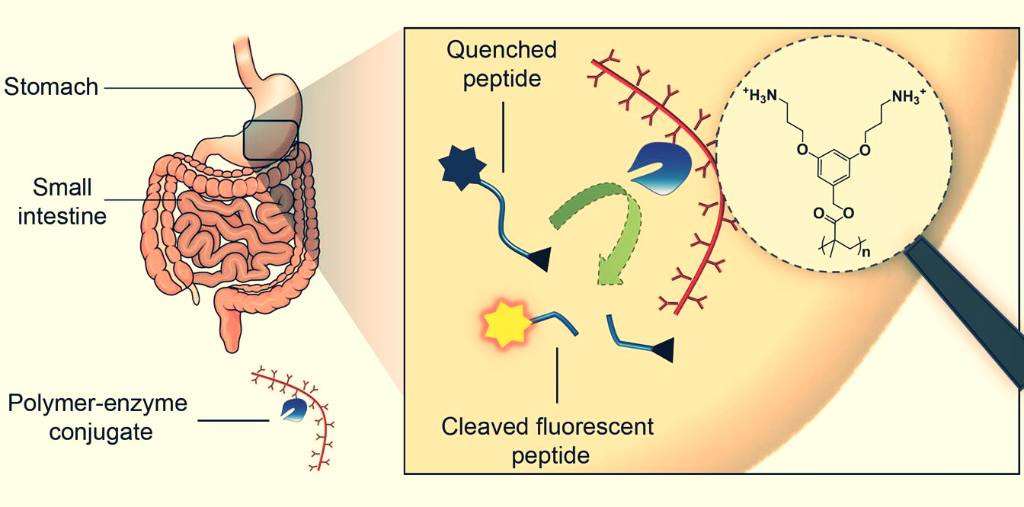
The gastrointestinal system represents a particularly harsh milieu in which proteins are degraded by proteases and extreme variations of pH (Fuhrmann et al., Pharm Res (2014)). We have developed an innovative approach for the stabilisation of therapeutic enzymes after oral ingestion. For this, a novel non-invasive assay to evaluate oral exogenous enzymes in vivo was designed (Fuhrmann et al., Proc Natl Acad Sci (2011)). This assay represents the first-time description of the real time measurement of activity of therapeutic enzymes in the gastrointestinal tract using coeliac disease as model disease (i.e., an inflammatory disease of the small intestine (Pinier and Fuhrmann et al., Am J Gastroenterol (2010)).
We have recently discussed how polymer modification of therapeutic peptides and proteins may enhance their stability in the gastrointestinal tract (Fuhrmann et al., Curr Opin Colloid Interface Sci (2017)). Taking advantage of this principle, we have modified therapeutic enzyme with architecturally and functionally diverse polymers and evaluated them using our non-invasive in vivo imaging assay (Fuhrmann et al., Nat Chem (2013)). It was shown that modification of the endopeptidase induced stabilisation of the enzyme and unprecedented sustainment of the gastrointestinal activity in vivo. Our findings create a strong basis for the development of therapeutic enzymes for oral administration using simple and straightforward methods.